
Thermodynamic modeling
 The laboratory has a long experience in the thermodynamics of electrolyte solutions resulting in the development of numerous Gex as well as, more recently, of equation of state models : Fürst and Renon (1993), Zuo and Fürst (1998), Radia and al. (2008), Suong and al. 2012).
The laboratory has a long experience in the thermodynamics of electrolyte solutions resulting in the development of numerous Gex as well as, more recently, of equation of state models : Fürst and Renon (1993), Zuo and Fürst (1998), Radia and al. (2008), Suong and al. 2012).
In the case of complex systems, these studies are often associated to original speciation determination, an example being the case of water – alcanolamine – CO2 systems : Archane and al. (2011), Diab and al. 2012).
The laboratory also has expertise in the use and development of state SAFT-type equations ![]() (Statistical Associating Fluid Theory : Jackson and al.).
(Statistical Associating Fluid Theory : Jackson and al.).
An advantage of these equations of state is their abilities handling with the same model a wide variety of systems ranging from electrolyte to polymer solutions.
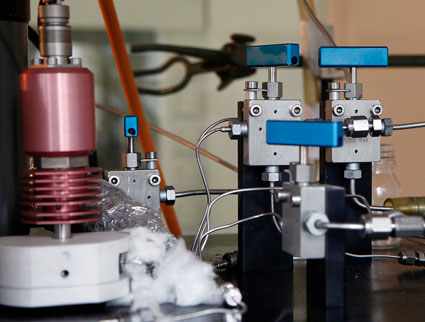 The studied systems are numerous: solutions of metallic complexes, acid gas absorption in amine aqueous solutions, mixed solvent electrolyte systems, systems involved in the production of H2 through the sulfur/iodine cycle, clathrate and semiclathrates stability conditions, ...
The studied systems are numerous: solutions of metallic complexes, acid gas absorption in amine aqueous solutions, mixed solvent electrolyte systems, systems involved in the production of H2 through the sulfur/iodine cycle, clathrate and semiclathrates stability conditions, ...
We also use the predictive models COSMO-RS et COSMO-SAC for the prediction of phase equilibria of multi-component mixtures of oxygen molecules (biofuels, food).
Contact : Patrice Paricaud, lecturer