Chemistry of nanomaterials
 The activity of the GDP group in mineral chemistry and nanomaterials takes over from that of the SCPI group at the École des Mines de Paris which ENSTA hosted for a dozen years. This activity is devoted to studying (co)precipitation in aqueous solutions.
The activity of the GDP group in mineral chemistry and nanomaterials takes over from that of the SCPI group at the École des Mines de Paris which ENSTA hosted for a dozen years. This activity is devoted to studying (co)precipitation in aqueous solutions.
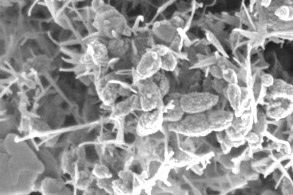
Work focuses on the synthesis of metallic (hydr)oxides with particle sizes ranging from a few nanometers to several microns. During recent years, Al, Si, Ti, Fe, Co, Ni, Cu, Zn, Y, Hf and Ce (hydr)oxides particles (as well as some doped or mixed compositions) were synthesized for applications in cosmetics, nanomedicine, batteries, photocatalysis...
Our objectives consist of providing robust tools to control particle size and morphology, with various industrial constraints in mind. In order to stick to industrially, economically and environmentally acceptable processes, we favour aqueous chemistry and emphasize the control over particle size and morphology thanks to simple physico-chemical parameters (pH, temperature...) and processes (double jet or homogeneous precipitation). Often the desired particles are not directly precipitated; instead a solid precursor (i.e., an amorphe) which is subsequently crystallized is produced. Uncoupling of amorphous precipitation and crystallization stages of particle preparation allowing the use of different physico-chemical conditions at each stage has been shown to be critical in controlling the nature and characteristics of the final particles.
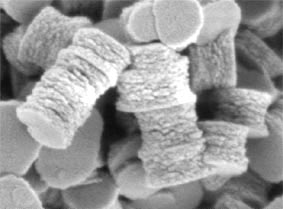
 We used this strategy in many studies (boehmite, zinc oxide, titanium dioxide, hafnium germanate...) to control particle size and shape from very simple amorphous precipitates.
The applications concerned are highly varied and have been the object of academic and industrial collaborations for over 10 years : particles for catalysis, photocatalysis or hydrogen photoproduction, for batteries, nanoparticles as ceramic precursors (thermoelectrics, fuel cells, ferroelectrics), photochromic particles for cosmetics, X-ray absorbers for nanomedicine...
We used this strategy in many studies (boehmite, zinc oxide, titanium dioxide, hafnium germanate...) to control particle size and shape from very simple amorphous precipitates.
The applications concerned are highly varied and have been the object of academic and industrial collaborations for over 10 years : particles for catalysis, photocatalysis or hydrogen photoproduction, for batteries, nanoparticles as ceramic precursors (thermoelectrics, fuel cells, ferroelectrics), photochromic particles for cosmetics, X-ray absorbers for nanomedicine...
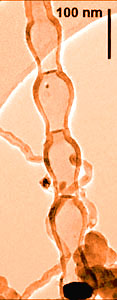
 The laboratory combines materials and industrial process research. The particles studied are principally metal hydroxides and oxides synthesized by precipitation of an amorphe followed by crystallization, by either double-jet precipitation at controlled pH or heat induced homogeneous precipitation. The lab is particularly interested in the role of pH on particle characteristics (size, morphology and crystallinity) in the double-jet precipitation of boehmite fibres, zinc oxide (with a morphology transition from non-starlike to starlike as a result of a strictly controlled pH change of only 1 unit) and nickel hydroxide.
The laboratory combines materials and industrial process research. The particles studied are principally metal hydroxides and oxides synthesized by precipitation of an amorphe followed by crystallization, by either double-jet precipitation at controlled pH or heat induced homogeneous precipitation. The lab is particularly interested in the role of pH on particle characteristics (size, morphology and crystallinity) in the double-jet precipitation of boehmite fibres, zinc oxide (with a morphology transition from non-starlike to starlike as a result of a strictly controlled pH change of only 1 unit) and nickel hydroxide.
The decoupling of the precipitation and crystallization steps has been exploited for the synthesis of boehmite nanoparticles (of varying crystallinity), hafnium germinate and titanium dioxide. Heat induced homogeneous precipitation can be separated into 3 variants : synthesis of yttrium or magnesium hydroxycarbonates by urea decomposition, destabilization of aminocomplexes (nickel, cobalt) and thermohydrolysis (titanium dioxide, tin oxide).
Partnerships and projects :
The studies at the laboratory are often conducted through direct collaboration with industry or as part of consortium in ANR or european projects.
- Ongoing ANR projects — team tasks :
- SYTCOM — nanothermite synthesis : coating of aluminum particles with nanoparticles of metal oxides.
- FERROENERGY — preparation of colloidal suspensions for the ferroelectric energy conversion.
- PARTOX — synthesis, characterization and cataloging of nanoparticles for toxicity studies.
- ONE — nanostructuring of nickel hydroxide for battery.
- TECTONIC — development of coprecipitated particles as precursors of ceramic-type proton conductors.
- POCANA — synthesis of boehmite particles coated with silica for use as fillers in polycarbonate.
- FP7 Projects :
- PHOTOMEM (Photocatalytic and membrane technology process for olive oil mill waste water treatment) — the synthesis of photocatalysts supported by magnetic particles.
- IDEAL CELL (Innovative Dual mEmbrAne fuel Cell) — synthesis of particles of cerium oxide and barium cerate doped to develop ceramics of this new concept of fuel cell.
Main academic collaborations :
- Paris Sorbonne Université — Laboratoire de réactivité des surfaces (synthesis of catalysts).
- École des Mines de Saint-Étienne (nanotoxicity).
- LNE (nanometrology).
- Centre PERSÉE (research about TiO2 and more generally nanomaterials for energy) of Mines ParisTech.
- CEMEF of Mines ParisTech.
- Centre des Matériaux (IDEAL CELL, TECTONIC, PARTOX + TiO2 activity and derivatives) of Mines ParisTech.
- IMT Mines Alès (POCANA).
- Institut de Chimie de la Matière Condensée de Bordeaux (ICMCB) (nickel hydroxide, inter Carnot ONE).
Main industrial collaborations :