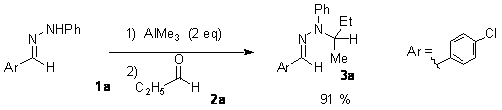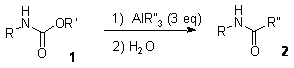
Aluminum chemistry
During our attempts to perform aldol type addition between hydrazone and aldehydes, we discovered that the addition of an aldehyde to the preformed complex of trimethylaluminum and hydrazone lead to an N-alkylated compound with transfer of the methyl group. Apart from the mechanistic interest of this reaction (analogous reaction with secondary amine instead of the hydrazone is not described), this coupling allows the preparation of highly hindered hydrazine derivatives...
This activation of an hydrazone by a trialkylalane has stimulated our attention on other potential conversion based on similar activation of N-H compounds. These trials finally lead us to disclose a useful fragmentation of carbamate using alanes. Carbamates are frequently used in synthesis as protecting groups for amines, their rather high stability has however constrained their used to several specific families such as BOC or benzyl type carbamate easily cleaved under acidic or reductive conditions. When carbamates derived from primary amines are heated togever with trialkylaluminum, highly selective formation of amides are observed by intramolecular alkyl group transfer from the aluminum to the carbonyl function.
Metathesis
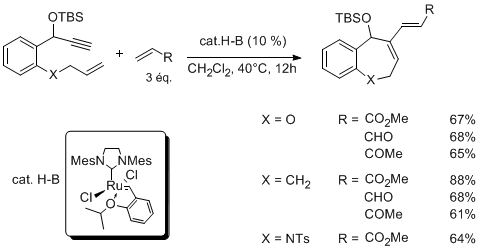
In 2003, we reported a tandem RCM-CM ene-yne-ene reaction. We have demonstrated that such cascades were efficient using electron-poor cross-metathesis partners, and we have studied the influence of steric hindrance at the propargylic position. Such tandem process was applied o the synthesis of the tricyclic squeleton of Paulitin,a sesquiterpene lactone, isolated from Ambrosia Artemisiaefolia, b
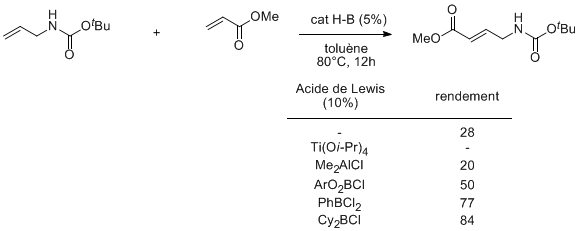
The use of allyl or homoallyl nitrogen derivatives in cross metathesis is often impeded by the poor kinetics of the couplings in relation with potential complexation of the nitrogen function to the catalyst. Indeed, we did not observe cross-coupling of various allylic and homoallylic carbamates using ruthenium catalyst (Hoveyda or Grubbs 2nd generation). Most noteworthy is the absence of reactivity observed when using the titanium catalysts well-known to promote the couplings of esters. We demonstrated that the use of boron Lewis acids as co-catalyst allows the formation of the expected product in good yields.
Oxidative processes
Various studies concerning metal-catalyzed processes have been done using Ugi-Smiles adducts as starting materials.
Publications
- « New trimethylaluminum induced Mannich type reaction of hydrazones », Laurent El Kaïm, Laurence Grimaud, Yannick Perroux, Cornelia Tirla, J. Org. Chem., 2003, 68, 8733-8735.
- « First carbamates conversion to amides by simple alkyl group trnsfer from trialkylalanes », L. El Kaïm, L. Grimaud, A. Lee, Y. Perroux, C. Tirla, Organic Letters, 2004, 6, 381-383.
- S« elective Domino Ring-Closing Metathesis-Cross-Metathesis Reactions between Enynes and Electron-deficient Alkenes », Royer F., Vilain C., EL Kaïm L., Grimaud L., Organic Lett. 2003, 5, 2007-2009.
- « Towards the Synthesis of Paulitin : New insights into Enyne-Metathesis Mechanism », Vedrenne E., Royer F., Oble J., El Kaïm L., Grimaud L., Synlett 2005, 15, 2379-2381.
- « Dramatic effect of boron-based Lewis acid in cross-metathesis reactions », Vedrenne E., Dupont H., Oualef S., El Kaïm L., Grimaud L., Synlett 2005, 15, 670-672.
- « Intramolecular Kulinkovich-de Meijere reactions of various disubstituted alkenes bearing amide groups », Madelaine C., Ouhamou N., Chiaroni A., Vedrenne E., Grimaud L., Six Y., Tetrahedron 2008 64, 8878-8898.
- « New Ugi-Smiles-metathesis strategy towards the synthesis of pyrimido azepines », L. El Kaïm, M. Gizolme, L. Grimaud, J. Oble, J. Org. Chem., 2007, 72, 5835-5838.
- « New indolizines template from the Ugi reaction », L. El Kaïm, M. Gizolme, L. Grimaud, Synlett, 2007, 227-230.
- « Ugi-Smiles access to quinoxalines derivatives », J. Oble, L. El Kaïm, M. Gizzi, and L. Grimaud, Heterocycles, 2007, 73, 503-517.
- « New MCR-Heck-isomerization Cascade towards Indole », Laurent El Kaïm, Marion Gizzi, Laurence Grimaud, Org. Lett. 2008, 10, 3417-3419.
- « New palladium-catalyzed aerobic oxidative cleavage and cyclization of N-aryl peptide derivatives », Laurent El Kaïm, Rocío Gámez-Montaño, Laurence Grimaud, Tannya Ibarra-Rivera, J. Chem. Soc., Chem. Commun. 2008, 1350-1352.
- « Towards new pyrrolo[2,3-d]pyrimidines scaffolds », El Kaïm Laurent, Grimaud Laurence, Wagschal Simon J. Org. Chem. 2010, 5343-5346.
- « Three-component Fischer indole synthesis », Laurent El Kaïm, Laurence Grimaud, Caroline Ronsseray, Synlett 2010, accepted.