Hydrazone chemistry
We have been interested in hydrazone chemistry for a long time, and various axes have been explored.
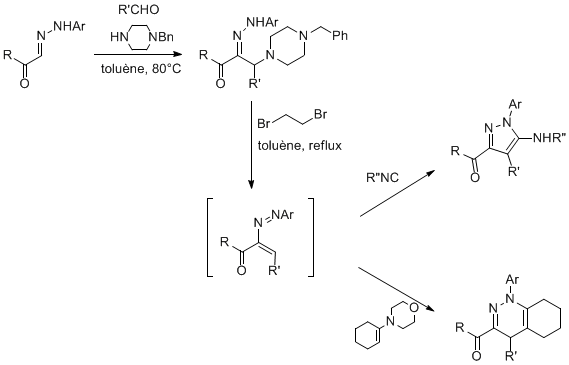
For instance, we have developed a new Mannich-type coupling involving hydrazones as the nucleophilic partner. Based on the former Keil and Reid work (1965), we developed a very effcient thre-component access to a-aminohydrazones which are stable precursor of azoalkenes. The use of N-benzyl piperazine allows efficient couplings whatever the aldehyde used and some mild conditions for the azoalkene generation. These heterodienes could be trapped in various cycloaddition reactions -[4+1] and [4+2]- to form different heterocyclic scaffolds.
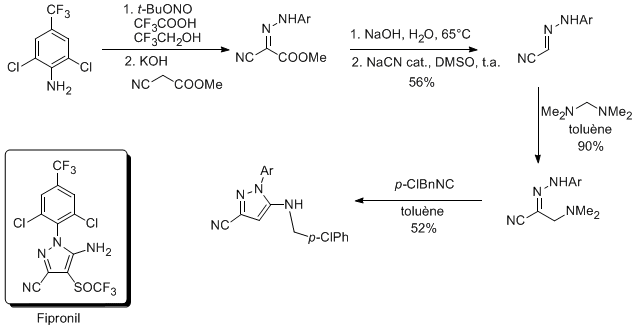
In collaboration with Aventis-Agro, the cascade Mannich reaction-[4+1]cycloaddtion was applied to the synthesis of a Fipronil precursor.
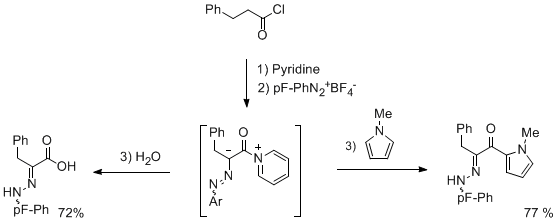
In connexion with our study on the reactivity of azoalkene, we became interested by the analogous behavior of azoketenes derivatives. We have disclosed a direct mild conversion of acyl chloride into azoketene derivatives, under pyridine activation in the presence of a diazonium salt.The synthetic interest of this approach was demonstrated by trapping the intermediate with different nucleophiles (water, alcohols, nucleophilic aromatic compounds...).
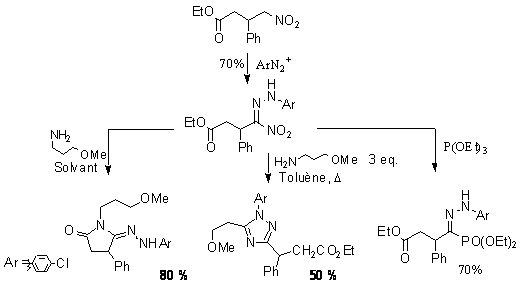
The use of nitro compounds as synthetic intermediates has triggered many efficient synthetic strategies for carbon-carbon bond formation or heterocyclic formation. The high acidity of the proton tethered to the nitro function allows the conversion of the nitro function under very mild conditions. The coupling with diazonium salts in particular is a high yielding process which have received little attention and have been poorly applied. We have used these couplings for simple two-step preparations of hydrazono phosphonate and amidrazone from nitro derivative. In the case of amidrazone, further cyclization to triazole give even greater value to this simple conversion.
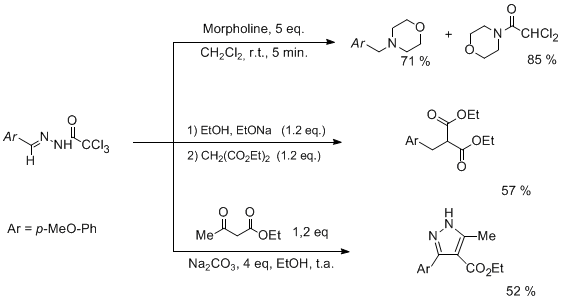
Trichloroacylhydrazones are easily prepared by condensing trichloroacylhydrazides with a carbonyl compounds. These compounds never studied before, have displayed in our hand a very puzzling chemical behavior. Though most of the compounds prepared are stable solids (easily stored several months without any decomposition), they appeared to be very sensitive to mild basic medium being transformed in highly reactive inetrmediates. Most noteworthy is their hability to alkylate various nucleophiles (amines, malonates...) with a reactivity pattern close to the one observed with diazocompounds.
Publications
- « A New Straightforward Formation of Aminopyrazoles from Isocyanides », V. Atlan, C. Buron, L. El Kaïm,, Synlett, 2000, 489-490.
- « New Versatile Approach to a-Hydrazonoesters and Aminoacids Derivatives through a Modified Japp-Klingemann Reaction », V. Atlan, L. El Kaïm, C. Supiot, Chem. Comm., 2000, 1385-1386.
- « The use of hydrazones for efficient Mannich type coupling with aldehydes and secondary amines », V. Atlan, H. Bienaymé, L. El Kaïm, A. Majee, J. Chem. Soc, Chem. Commun., 2000, 1585-1586.
- « The Mannich reaction of Hydrazones Amenable to Solid Phase Synthesis : A powerful Tool for Heterocycle Preparation », V. Atlan, L. El Kaïm, L. Grimaud, N.K. Jana, A. Majee, SYNLETT, 2002, 352-354.
- « Nitrohydrazones : versatile intermediates for phosphonate derivatives formation from primary nitro compounds », L. El Kaïm, L. Grimaud, N.K. Jana, C. Tirla, Tetrahedron Letters, 2002, 2037-2038.
- « Studies towards the synthesis of Fipronil analogues: improved decarboxylation of -hydrazonoacid derivatives », J.E. Ancel, L. El Kaïm, A. Gadras, L. Grimaud, N.K. Jana, Tetrahedron Letters, 2002, 8319-8322.
- « Amines addition to a-nitrohydrazones : application to amidrazones and triazoles formation » , L. El Kaïm, L. Grimaud, N.K. Jana, F. Mettetal, C. Tirla, Tetrahedron Letters, 2002, 43, 8925-8927.
- « The Mannich Reaction of Hydrazones : Improved Reactivity under Solvent-free Conditions », L. El Kaïm, L. Gautier, L. Grimaud,L. M. Harwood and V. Michaut, Green Chem., 2003, 5, 477 - 479.
- « A New Straightforward Formation of Aminopyrazoles from Isocyanides », V. Atlan, C. Buron, L. El Kaïm, Synlett, 2000, 489-490.
- « New insight into the azaenamine behavior of N-Arylhydrazones: first Aldol and improved Mannich reactions with unactivated aldehydes », Laurent El Kaïm, Laurent Gautier, Laurence Grimaud, Valérie Michaut, Synlett, 2003, 1844-1846.
- « New straightforward quinoline synthesis from the Mannich reaction of ?-ketohydrazones », V. Baillez, L. El Kaïm, V. Michaut, Synthetic Communication, 2004, 34, 109-118.
- « Three-component Fischer indole synthesis », Laurent El Kaïm, Laurence Grimaud, Caroline Ronsseray, Synlett 2010, 2296-2298.
- « 1,2,4-Triazole synthesis via amidrazones », Laurent El Kaïm, Marion Gizzi, Laurence Grimaud, Synlett 2010, 1771-1774.